(This is part 3 in a series titled the Search for a Cure, click here for Part 1: Back to the future or here for Part 2: Coping with Biology)
(The following was written with a lot of help from Dr. Kalpana M. Merchant. Dr. Merchant has nearly 25 years of experience in drug discovery and development with a special emphasis on translational strategies that improve the success rate of drug development. Click here to for a recent interview with Dr. Merchant.
The Process
“You can chew all the celery you want, but without antibiotics, three quarters of us would not be here.” – Dr. House
Modern healthcare is built, in large part, on finding druggable solutions to treat diseases. However, making a drug is an incredibly challenging process that can take up to twenty years and come with an estimated total capitalized cost averaging nearly 2.6 billion dollars per drug. Yet, it has been critical to significantly extending and improving quality of life for billions by providing treatments for a wide range of diseases. So, it’s worth understanding how it is that a drug goes from an idea in someone’s head to a box of pills in your medicine cabinet.
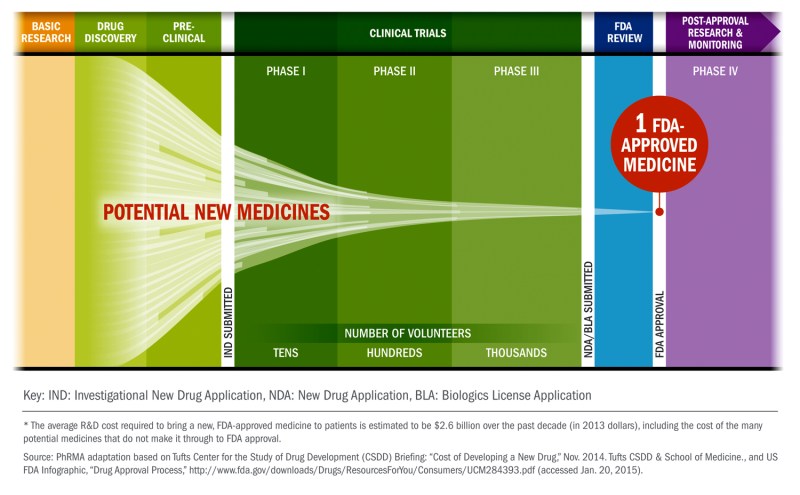
Let’s start with an overview…

That is the basics, but each step is much more complex and the details matter. Although the figure shows the process as a unidirectional flow of activities, in practice there are iterative learning loops at all stages to increase the success rate for delivering a safe and effective therapy. Below are explanations of the most critical steps in drug development, each step represents a key hurdle that needs to be crossed for a drug to make it to patients.
Discovery and Target Validation
The first two steps are often inseparable. This stage is typically the domain of scientists working in academic research centers funded by government agencies (e.g., National Institutes of Health) or other non-profit entities. However, it is also becoming increasingly common for biotechnology and pharmaceutical companies to collaborate in this endeavor.
The primary goal of the “Discovery” step is to identify drug targets through studies that provide insights into disease mechanisms. It often starts with the formulation of a hypothesis by generating and/or mining large-scale human data (e.g., genetics, electronic medical records, etc.). The hypothesis is then iteratively tested in a laboratory through cell and animal models. These model systems provide the means to identify mechanisms that cause or ameliorate the disease process or symptoms. Often, these mechanisms are not suitable as drug targets, requiring further studies into the biological processes to precisely identify druggable targets.
Scientists then publish these research findings in peer-reviewed journals. This is usually when findings make their way to the popular press, leading to headlines like “Major Breakthrough, Scientists Discover New Way to Treat Alzheimer’s“. Pharmaceutical companies of all sizes often rely on such publications to initiate drug discovery projects after confirming the basic findings in their own laboratories and relying on their own experience to determine if the mechanism discovered can be targeted by a drug. It is also not unusual for new companies to be founded on such discoveries. On average it takes 12-15 years to get from here to market.
Finding the Initial Molecules (“hits”)
This step begins with the development of screening assays. Assays are laboratory tests used to identify molecules that can modulate the desired target appropriately (i.e., increase or decrease its activity or expression) in a test tube and/or in cells. These assays screen 100s -1000s of compounds to identify initial “hits”. The hits rarely possess desired drug-like properties such as potency, target selectivity, solubility, cell penetration, etc. Medicinal chemists combine the biological data on the hits with structural knowledge to modify the compounds and generate leads that have the potential to deliver drugs. This step typically takes 6-12 months to complete.
Optimizing the Leads
This step aims to deliver preclinical drug candidates suitable for further development. It requires a multi-disciplinary team where medicinal chemists work collaboratively with biologists, pharmacologists, drug-distribution scientists, and others. Together they make molecules that can: (a) penetrate the intended organ such as the brain sufficiently through the chosen route of administration, (b) activate the target in the desired way and (c) provide evidence of efficacy or biomarker changes in animal models. Not surprisingly, this is a relatively labor-intensive step. It typically takes 12-18 months to go from leads to identifying a handful (typically 3-6) of preclinical candidates for further testing.

Preclinical Development
The primary goal of this stage is to conduct in vivo efficacy and toxicology studies to generate a data package that is submitted to a regulatory authority (e.g., FDA) for approval as an Investigational New Drug (IND). The main purpose of these studies is to demonstrate that the drug candidate produces efficacy at a dose that is appropriately lower than the dose at which any adverse events or toxicity may be observed (this is termed margin of safety). Toxicology studies are typically performed in two animal species. It is also critical to demonstrate the ability to manufacture and formulate the drug candidate suitable for human clinical studies. It is not unusual for small start-up companies to seek a pharmaceutical company as a partner at the end of this stage since clinical development is the most-resource intensive phase of the process. Due to the variable nature of this stage it can take anywhere from 1-3 years to complete.
Clinical Development
Typical stages of clinical development are designated as Phase 1, 2 and 3. With rare exceptions, all clinical testing is done under “double-blind” and placebo-controlled conditions where patients as well as physicians and others monitoring the drug’s effects are unaware of the treatment group that each subject is assigned to.
Phase 1 generally begins in healthy volunteers to evaluate the pharmacokinetic properties (time course of drug concentration in the blood) and establish its safety and tolerability at a range of doses after both single and repeated administrations. The number of subjects typically varies from 20-80. It is becoming increasingly common to extend Phase 1 testing into patients in the so-called Phase 1b trials to demonstrate safety, characterize pharmacokinetics, as well as assess whether the drug is able to engage and/or modulate the intended target using a biomarker. Where possible, the initial detection of a signal that might be predictive of clinical effectiveness (e.g., glucose levels in diabetics or cognitive/motor tests for Alzheimer’s or Parkinson’s disease) is highly desirable. These studies should allow to narrow the dose range of the drug candidate for further efficacy testing in Phase 2. In some cases, if the results of phase 1b are particularly promising, and Phase 2 trial duration may be long (e.g., for slowing progression of the disease), the drug candidates may progress directly to Phase 3. Typical duration: 4-6 months.
The goal of Phase 2 is to test the drug in a larger number of patients (typically 30-50 in each the placebo and drug arms) to continue to establish safety and tolerability while assessing clinical effectiveness on end-points approved by regulatory authorities. It is also highly desirable to collect supplemental biomarker data predictive of clinical efficacy such that at the end of Phase 2, there is sufficient data to make an informed decision on whether or not to proceed to the highly resource-intensive Phase 3 trials. Typical duration: 1-2 years.
Phase 3 trials are designed by the sponsoring company in consultation with a regulatory authority (e.g., FDA). They are typically conducted in anywhere from a few hundred to up to three thousand patients per treatment arm. They are designed to test the effectiveness of the drug while gathering information on safety and tolerability in the intended patient populations. Typical duration: 3-4 years.
New Drug Application (NDA) Submission and Regulatory Approval.
This is the formal step that the sponsor of a drug takes to ask the FDA for drug approval in the United States. An NDA application contains all animal and human data, analyses of the data, as well as information on drug manufacturing. It typically takes up to ten months to be evaluated by FDA scientists.

To Market…Finally
At this point, pharmaceutical companies manufacture and market the drug. The goal of the company is to price it competitively but adequately to maximize profits while the drug is still protected by patents. Often a Phase 4 study will also be run that encompasses specific post-market monitoring, if required by regulatory authorities.
Now, finally, the long road to market is complete.
That is the process in a nutshell. But it is important to state that this is a general overview and that many of the steps mentioned were simplified versions of an even more elaborate processes.. That said, now that we have some understanding of what the process of bringing a new therapy to patients is like, we can start talking about what those new therapies will be in part 4 of this series…
The Search for a Cure
Part 3: Understanding Drug Development
Coming up in this series...(tentative schedule)
Part 4: Expert survey of disease-modifying drug targets
Part 5: Restorative treatments on the horizon
Part 6: Obstacles to Overcome
Part 7: The Bigger Problems
Part 8: Technology to the Rescue
Part 9: The Promise of Precision Medicine
Part 10: The Role of Patients
Part 11: Where Hope Springs
Part 12: The End of PD – Not with a bang, but a whimper
Other useful content…
(click here, here and here for a more detailed understanding of the drug development process.)

Ben,
One missing yet very important issue in the drug development is that there is no way (as far as I know) for the FDA to approve medications that were used safely in Europe for decades but for some reason never came to US. One such drug is ambroxol – the medication that shows a lot of promise in Parkinson treatment. Ambroxol is readily available over the counter in Britain, Germany, and many other countries but not in US. Since the patent for it is expired there is nobody who would spend the money to go through the hoops.
Felix